Prototype of a clinical CT device combines dark-field X-ray and conventional technology
New technology for clinical CT scans

Computed tomography (CT) is one of the most important clinical methods for precise and fast diagnostics. By combining multiple X-ray images three-dimensional images of the patient are generated.
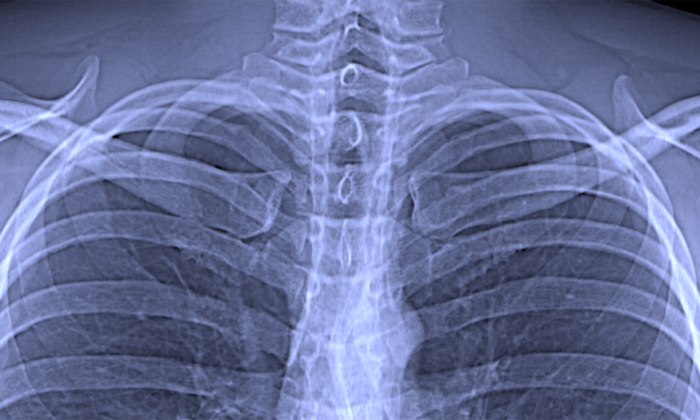
With dark-field imaging now additional information on fine tissue structures, in particular in the lung, is accessible. Until now, technical challenges have prevented the integration of this new technology into clinical CT scanners to examine patients.
A team of researchers working with Franz Pfeiffer, Professor for Biomedical Physics and Director of the Munich Institute of Biomedical Engineering at TUM, has now developed a CT scanner that combines both X-ray technologies.
“For the first time, we showed that dark-field X-ray technology can also be integrated into a clinical CT scanner. Although this technology is in its early stages, pre-clinical studies with mice have demonstrated clear benefits from dark-field CT scans, especially for capturing images of lung tissue,” says Franz Pfeiffer, who headed the study.
The new CT prototype has already been used successfully with a thorax phantom, a model of a human upper body, and is large enough for the intended applications with real patients.
Conventional X-ray imaging
With conventional X-ray equipment, the X-rays are attenuated by the intervening tissue as they travel from the source to the detector. This effect is used to produce images based on the varying degrees of attenuation associated with different tissue types and structures. That is why bones and similar structures, which have a stronger attenuating effect, appear white in X-rays, while more transparent tissue types such as the lung produce darker images.
Dark-field X-ray imaging
Dark-field imaging, by contrast, makes use of the small-angle scattering of the X-rays. When the X-rays interact with materials of different densities such as the interface between lung tissue and air, they are scattered. The analysis of this scattering effect yields additional information on very fine tissue structures, which is otherwise not accessible with conventional X-ray images.
Grating technology for dark-field imaging
To detect the scattering of the X-ray radiation, a set of three optical gratings is required. They are placed between the X-ray source and detector. When X-rays pass through these gratings, a characteristic pattern is produced at the detector. When a sample or person is then positioned in the beam path, this characteristic pattern is changed. These deviations are then used to analyze the structure of the sample or the person’s tissue.
New hardware and software for dark-field CT
The implementation of the dark-field method in a human-size CT scanner poses various technical challenges. Until now, this has limited dark-field CT devices to a scale much smaller than would be needed for human patients. Apart from the size, the fast rotation of the scan unit also creates special difficulties for the technical design.
The scanning unit of CT scanners, known as the gantry, rotates at very high speeds. This causes vibrations that affect the finely-tuned components in the interior of the device. Based on a detailed analysis of these vibrations, the team was able to use them to implement the required shift between the gratings needed for dark-field imaging. To analyze the scans, they developed new algorithms to filter out the vibration effects based on reference scans.
Additional information for clinical diagnostics
“With the dark-field CT prototype, we can capture conventional and dark-field X-ray images in a single scan. This yields additional information that could be used in the future not only to diagnose lung diseases, but also to differentiate between various types of kidney stones and tissue deposits,” says Manuel Viermetz, one of the two first authors of the study. As the next step, the researchers plan to further optimize the dark-field CT prototype and prepare for the first scans of human patients.
Dark-field computed tomography reaches the human scale.
Manuel Viermetz, Nikolai Gustschin, Clemens Schmid, Jakob Haeusele, Maximilian von Teuffenbach, Pascal Meyer, Frank Bergner, Tobias Lasser, Roland Proksa, Thomas Koehler andFranz Pfeiffer. PNAS, Februar 2022. DOI: https://doi.org/10.1073/pnas.2118799119
- Co-author Thomas Koehler (Philips Research) is a Rudolf Diesel Industry Fellow of the TUM Institute for Advanced Study (TUM-IAS), which is funded in part by the Excellence Initiative of the German federal and state governments and by the EU Marie Curie COFUND program.
- The work was supported by the European Research Council through an Advanced Grant and Philips GmbH Market DACH.
- A portion of the work was carried out in collaboration with the Karlsruhe Nano Micro Facility (KNMF), a Helmholtz research infrastructure facility at the Karlsruhe Institute of Technology (KIT).
- Prof. Franz Pfeiffer is the Director of the Munich Institute of Biomedical Engineering (MIBE). MIBE is an Integrative Research Institute (IRI) within the Technical University of Munich (TUM). At MIBE, researchers specializing in medicine, the natural sciences, and engineering join forces to develop new methods for diagnosing or treating diseases. They also work on improving technologies that compensate for physical disabilities. The activities cover the entire development process – from the study of basic scientific principles through to their application in new medical devices, medicines and software.
Technical University of Munich
Corporate Communications Center
- Carolin Lerch
- carolin.lerch@tum.de
- presse@tum.de
- Teamwebsite
Contacts to this article:
Prof. Dr. Franz Pfeiffer
Technical University of Munich
Chair of Biomedical Physics
Tel: +49 89 289 12551
franz.pfeiffer@tum.de
https://www.bioengineering.tum.de/
![[Translate to en:] Prof. Dr. Franz Pfeiffer am Dunkelfeld-Computertomograph. [Translate to en:] Prof. Dr. Franz Pfeiffer am Dunkelfeld-Computertomograph.](/fileadmin/_processed_/d/2/csm_20220121_Pfeiffer_Medical_Imaging_AE_-195_5f45e33338.jpg)
![[Translate to en:] Thorax Phantom in einem Dunkelfeld-Computertomographen. [Translate to en:] Thorax Phantom in einem Dunkelfeld-Computertomographen.](/fileadmin/_processed_/7/d/csm_20220121_Pfeiffer_Medical_Imaging_AE_-1050_fd4a45d8de.jpg)

![[Translate to en:] Eine Kombination von Dunkelfeld- und konventionellem Röntgenbild ermöglicht eine klare Unterscheidung zwischen gesundem und emphysematösem Gewebe sowie eine Beurteilung der regionalen Verteilung der Krankheit. Anhand solcher Bilder k A combination of dark-field and conventional transmission information allows for a clear distinction of healthy versus emphysematous tissue and an assessment of the regional distribution of the disease. From such images, a doctor might in future not only see if a patient is diseased but also which parts of the lung are affected and how much. Picture: Simone Schleede / TUM](/fileadmin/_processed_/6/d/csm_121022_Dunkelfeld_900_28fbd8d6cb.jpg)