Biophysicists construct complex hybrid structures using DNA and proteins
Designer proteins fold DNA
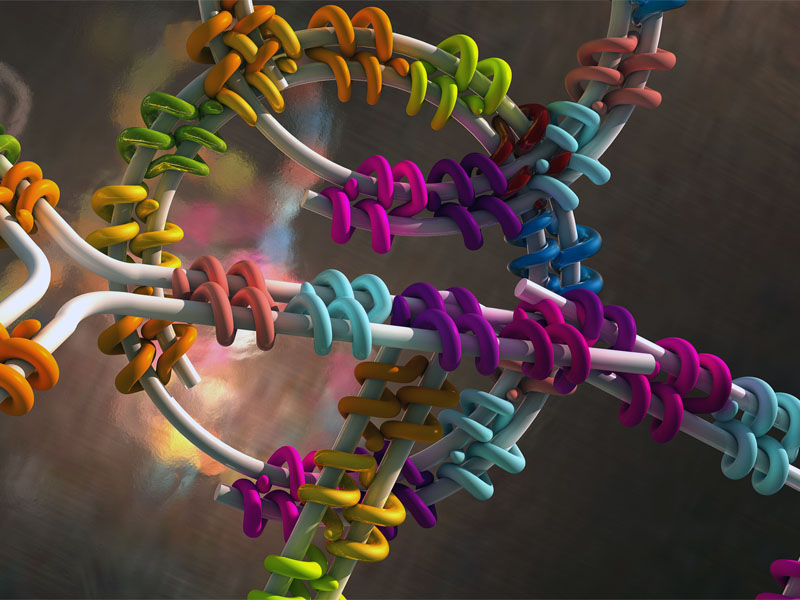
Desoxyribonucleic acid, better known by its abbreviation DNA, carries our genetic information. But to Prof. Hendrik Dietz and Florian Praetorius from TUM, DNA is also an excellent building material for nanostructures. Folding DNA to create three-dimensional shapes using a technique known as "DNA origami" is a long-established method in this context.
But there are limits to this approach, explains Dietz. The "construction work" always takes place outside of biological systems and many components must be chemically synthesized. "Creating user-defined structures in sizes on the order of 10 to 100 nanometers inside a cell remains a great challenge," he adds. Their newly developed technique now allows the researchers to use proteins to fold double-stranded DNA into desired three-dimensional shapes. Here, both the DNA and the required proteins can be genetically encoded and produced inside cells.
Proteins act as staples
Designed "staple proteins" based on TAL effectors are the key to the method. TAL effectors are produced in nature by certain bacteria that infect plants and are able to bind to specific sequences in the plant DNA, thereby neutralizing the plant's defense mechanisms. "We've constructed variants of the TAL proteins which simultaneously recognize two custom target sequences at different sites in the DNA and then basically staple them together," says Dietz. "This was exactly the property we needed: proteins that can staple DNA together."
The second component of the system is a DNA double strand containing multiple binding sequences that can be recognized and linked by a set of different staple proteins. "In the simplest case a loop can be created by binding two points to one another," Praetorius explains. "When several of these binding sites exist in the DNA, it's possible to build more complex shapes." An essential aspect of the researcher's work was therefore determining a set of rules for arranging the staple proteins themselves and how to distribute the binding sequences on the DNA double strand in order to create the desired form.
New tools for fundamental research
What's more, the staple proteins serve as anchor points for additional proteins: A method referred to as genetic fusion can be used to attach any functional protein domain desired. The hybrid structures made of DNA and proteins then function as a three-dimensional framework which can put the other protein domains into a particular spatial position. All the building blocks for the DNA protein hybrid structures can be produced by the cell itself and then assemble themselves autonomously. The researchers were able to produce the hybrids in environments resembling cells starting from genetic information. "There is a fairly high probability that this will also work in actual cells," says Dietz.
The new method paves the way for controlling the spatial arrangement of molecules in living systems, which allows probing fundamental processes. For example, it's assumed that the spatial arrangement of the genome has a substantial influence on which genes can be read and how efficient the reading process is. The intentional creation of loops using TAL-DNA hybrid structures in genomic DNA may provide a tool for investigating such processes. It would also be possible to geometrically position a series of proteins inside and outside the cell in custom ways in order to investigate the influence of spatial proximity for example on information processing in the cell. The spatial proximity of certain enzymes could also make processes in biotechnology more efficient. Lastly, it would also be conceivable to utilize protein-DNA hybrid structures for example to better stimulate the immune response of cells, which can depend on the precise geometrical arrangement of multiple antigens.
---
The project was financed by the German Federal Ministry of Education and Research as part of the ERANET SynBio program "BioOrigami", as well as by the Gottfried-Wilhelm-Leibniz program of the German Research Foundation (DFG) and by the Excellence Clusters CIPSM and NIM.
Publication:
F. Praetorius and H. Dietz, ‘Self-assembly of genetically encoded DNA-protein hybrid nanoscale shapes’; Science, (2017), doi: 10.1126/science.aam5488 (Cover article)
Highresolution picture:
Contact:
Prof. Hendrik Dietz
Technical University of Munich
+49 (0) 89 289-11615
dietz@tum.de
dietzlab.org
Florian Praetorius
Technical University of Munich
+49 (89) 289 - 11622
florian.praetorius@mytum.de